How to calculate carbon dioxide equivalent emissions from different GHG sources?
Green House Gases (GHGs) are those that can absorb and emit infrared radiation. There are six Green House Gases as identified under the Kyoto protocol. These are:- Carbon dioxide (CO2), Methane (CH4), Nitrous oxide (N2O), Hydrofluorocarbons (HFCs), Perfluorocarbons (PFCs); and Sulphur hexafluoride (SF6). While creating the GHG inventory of the organization, there is a need to calculate all the six GHGs e.g. GHG emissions from the burning of fossil fuels, methane emission from anaerobic digestion of organic matter (Spent wash treatment in anaerobic digester in molasses based distillery), CO2 emission from use of fire extinguishers during actual fire conditions or emergency preparedness programs/training in the organization, etc.
Clause 4.3.3 of ISO 14064-1 specifications suggests that organization shall select and use quantification methodologies that will reasonably minimize uncertainty and yield accurate, consistent and reproducible results. ISO 14064-1 specifies that organization shall calculate their GHG emission as per six greenhouse gases separately. The same method is given in following schematic;
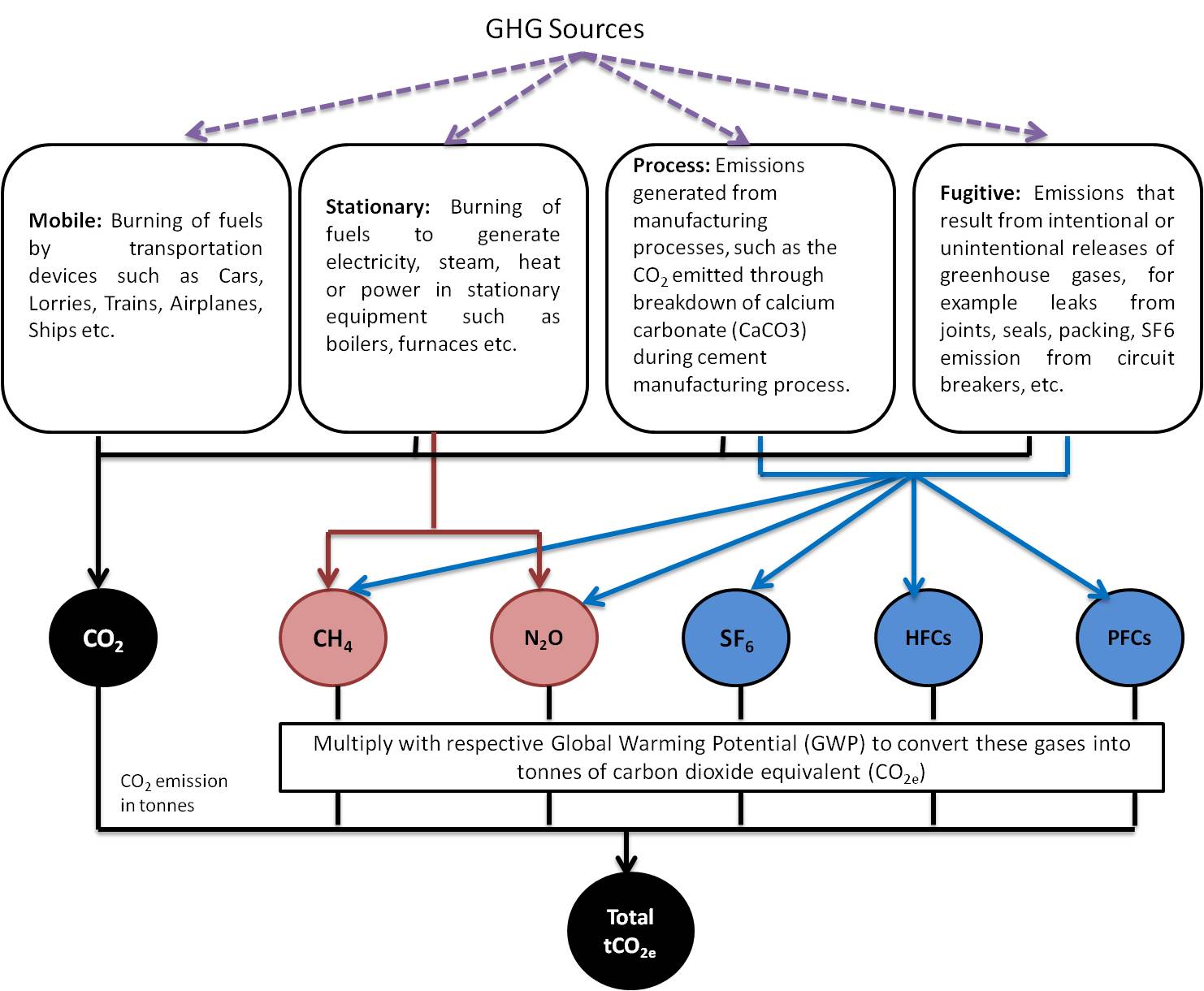
Therefore, whatever the source of Green House Gasses and the calculation methodology, the resulting value should be in mass unit of Carbon dioxide equivalent form. By multiplying the resulting value of gas other than CO2 with the respective Global Warming Potentials (GWP) will result in CO2 equivalent form. The concept of global warming potential (GWP) has been developed in order to enable comparison of the ability of different GHGs to trap heat in the atmosphere (Read more on GWP here). Following table shows GWP of different GHGs;
|
GHGs |
GWP |
| Carbon dioxide CO2 |
1 |
| Methane CH4 |
21 |
| Nitrous oxide N2O |
310 |
| Sulphur hexafluoride SF6 |
23900 |
| HFC | |
| PFC |
Following examples will provide calculation methods as per the GHG protocol and ISO 14064-1;
Example 1: GHG emission from the burning of diesel in stationary source i.e. DG sets.
Burning of fuel in stationary source emits Methane and Nitrous oxide. To understand the further calculation process, let’s take 100 liters of diesel consumption in the DG set. The calculation would be;
- CO2 emission = Fossil fuel consumption in volume unit X CO2 emission factor (Ton per volume unit)
- CH4 emission = Fossil fuel consumption in volume unit X CH4 emission factor (Ton per volume unit)
- N2O emission = Fossil fuel consumption in volume unit X N2O emission factor (Ton per volume unit)
Total GHG emission (in tCO2 eq) = (CO2 emission) + (CH4 emission X 21) + (N2O emission X 310)
- CO2 emission = 100 X 0.00265
- CH4 emission = 100 X 0.00000036
- N2O emission = 100 X 0.000000021
Total GHG emission (in tCO2 eq) = 0.265299393 + (0.000035819 X 21) + (0.00000215 X 310)
Total GHG emission (in tCO2 eq) = 0.2667
Example 2: GHG emission from the use of R-134a [HFC – C2H2F4 (CH2FCF3)] from the air conditioning system
Conventional air conditioning systems uses HFC based referents which might leak HFC gas into the atmosphere. Let’s consider 100 kg of HFC leaked from the air conditioning systems, then how can we calculate GHG emission in CO2 equivalent form? The answer is here;
R-134a (HFC) = 100 Kg
Global Warming Potential of R-134a = 1300 t CO2 e per ton of R-134a
Total GHG emission (in tCO2 eq) = (100/1000) X 1300
Total GHG emission (in tCO2 eq) = 130
Note: For GHG emission factors, please refer IPCC GHG EF database


How much Carbon Dioxide is produced by 1 liter of diesel for generation electricity.
Fact : To produce 100 units through 100 KW diesel generator, 12 liters of diesel is used.
So how much CO2 is produced by burning 1 liter.
Please send the explanation and facts
For 12 litres of diesel consumption in stationary GHG emission source like DG set will release 0.03184 tons of carbon dioxide equivalent emission (Diesel emission factor is 0.002653 tCO2/Lit. This is based on diesel density of 0.833 kg/Lit and 43 TJ/Gg of NCV). Diesel density value is sourced from Indian Oil website.
Note 1: I haven’t considered CH4 and N2O emission.
Note 2: Diesel density along with carbon content and NCV determines the value of GHG emission factor.
Thank you very much for this very valuable knowledge dear author. It shed some light. I was ask to compute for the GHG emission for the propose DG set for our client, when all my notes were gone. This helps me, I was able to review again on the concept and the process. Please post for more examples. Again, thank you.
Why in my notes, the Diesel emission factor of CO2 is 2.7126 kgCO2/liter or 0.0027126 kgCO2/mL and not the same as what you have given in the above article which is 0.002653 tCO2/L?
i really like your articles, because they are providing me with the necessary info neede in my bid to provide carbon inventory for some organisations in nigeria. Am hoping to sign up with you guys sooner.
Thanks Igono for your valuable comment. Stay tuned with us for more interesting information. To receive email updates, you can subscribe via your email id (You need to verify it after signing up). In addition, you can ‘Like’ us on Facebook. We update our Facebook page regularly. Thanks again!
Sailesh.. Even I am confused in the same thing.
actually I need trhe co2 emsission from D.G Set.
say for e.g: 100lt. of diesel will consume ……..co2 emission.
Kindly reply
Hi, just use following GHG emission factor for diesel i.e. 0.00265 tCO2/Lit. Multiply this value with the diesel consumption value in liters. You will get carbon emission value in tons.
In addition,calculate CH4 and N2O emissions separately. You will have to multiply resulting values with relevant global warming potential. Add this to co2 emisisons and you will get carbon dioxide equivalent emissions.
shailesh can you please tell me values of co2 emission factors of LPG and NATURAL GAS
Hi, I do not have numbers readily available at this moment. Please visit the following link to get the emission factors http://www.ghgprotocol.org/calculation-tools/all-tools (You will have to download excel sheet and get the EF).
Please explain how is the amount of HCF leakage from Air conditioning system determined in actual scenario.
Generally organizations have HFC based cooling systems like chillers, air conditioners, cold storages, etc. There is no simple method to quantify the leakage of HFC in these cooling systems physically. They refill/top-up the HFC based cooling system based on the requirements and operational conditions. Therefore, you can ask organization to show bills/Pay-receipt for HFC top -up. This receipt act as the evidence for HFC leakage. Every receipt has information on the quantity of HFC refilled.
Kindly clarify,Guidance on GHG emissions from stationary speaks only about CO2 emissions(as they are major) although the emissions include CH4 and NO2.Also provide information on which parameters to be considered for emissions from Transportation(by employees)
It is important to measure/calculate CH4 and N2O emissions as per the requirements of ISO 14064-1 and GHG protocol. So, don’t give them less preference. Examples of Stationary sources are DG sets, Machinery, etc. Multiply GHG activity data with relevant GHG emission factors given by IPCC/EPA and you will get the value of GHG emissions For the quantification of GHG emission from vehicles, you can apply the same procedure. GHG protocol provided GHG emission factors for the transportation. It comes under scope 3 GHG emission depends on the boundary selected. There are two ways to calculate GHG emission from transportation. One is to use distance based emission factors and second one is using fuel based emission factor. Based on the availability of GHG activity data i.e. distance based data or data on total fuel consumption can be used.
Thanks a lot.
i want to know CO2 emission factors for India for electricity, Gas, Petrol, indoor air
For fossil fuel, use following link https://greencleanguide.com/2011/10/25/calculate-co2-emissions-from-fossil-fuel-combustion/
For electricity;
For northern grid of India = 0.94 tCO2/MWh
For southern grid of India = 0.91 tCO2/MWh
This has been very helpful but for our purposes confusing. Firstly trying to figure out where to start.
We do Electronic Recycling and we are trying to work out per ton of E-Waste, what the CO2e savings are.
Please help
Hi, For Electronic Waste Recycling Unit,you can start with identifying organizational boundary first. Post that, various greenhouse gas (GHG) sources can be identified within the selected boundary such as Mobile sources (Vehicles, etc), Stationary sources (DG sets for back up power, or any other fuel based equipment that is being used there, etc), Process based sources (If you have certain processes that release GHGs), etc. Purchased electricity from the local utility company is also need to be included. Once the identification of GHG sources is done, you can collect activity data like fuel consumption, electricity consumption, other raw material consumptions (specifically for the process), etc. IPCC has given emission factors for most of the fuels. GHG protocol website also provide details on the GHG emission factors. Depends on the selected GHG quantification methodology, you can multiply activity data with the emission factor and can quantify GHG emissions.
Thank you
Dear sir,
I have searched many sites for calculations and emission factor values related to GHG calculations, however i found this post most useful related to my work. I really want to appreciate efforts of you and your team members.
Thank you and keep posting sir
Hi,
Could you support me in below mentioned calculation as if I check CO2 emission for Electricity as well as Diesel for same power generation (21MWH), it seems Electricity is emitting more CO2 than HSD.
DG Units Saved Per Month KWH 21000
HSD Consumption (A) in KLtrs 6
CO2 Factor for KLtrs (B) 2.585
CO2 Emission by HSD Ton/Yr [A*B] 15.51
Electricity Per Month KWH 21000
Electricity Per Month MWH (a) 21
CO2 Factor (b) 0.926
CO2 Emission by HSD Ton/Yr [a*b] 19.446
Hi Poonam,
Usually, DG set generates 4 units (kWh of electrical energy) per lts of Diesel consumed. I don’t understand the rationale behind 21,000 kWh (21 MWh). To generate 21,000 kWh, about 5,250 lts of Diesel is required. Please provide more details and numbers on this particular case.
Note: Please note that the emission factor for electricity is actually an average grid emission factor. The power grid in India has electricity from all form of power generating sources.
Thanks.
GCG Team
Hi,
could you help me?
How to estimate national emission and how to estimate carbon emission in India from individual administrative regions.
Hi, please check ‘India’s First Biennial Update Report to the United Nations Framework Convention on Climate Change’ for more information. http://unfccc.int/resource/docs/natc/indbur1.pdf
Hi Shailesh,
You are really doing a nice job.
Can you please tell me where can I get calculation for different segments such as amount of plastic bags usage for 1 kg of CO2 emission, how much electricity in KW is equivalent to 1 Kg of CO2 emission.
Hello,
What is CO2 emission factor form a stationary source?
What is CH4 emission factor form a stationary source?
What is N2O emission factor form a stationary source?
Thank you
Really helpful responses to questions asked. Thank you.
Hello,
Based on the Vehicle Equipment type like BS2, BS3, BS4 etc., can you help me to calculate GHG (CO2e) for commercial vehicle. CO2 per Ltr of diesel or CO2 tonne / KM
Hello Sailesh,
I have a lot if the query to understand the calculation of Co2, basically, we are a manufacturer of gears cutting and doing the activity for GHG.
we need a calculation of the neat-cutting oil, how much CO2 carbon dioxide is produced by the one letter of neat-cutting oil.
we have replaced so many wet types (neat cutting oil) with a dry cutting machine. we need to calculate.